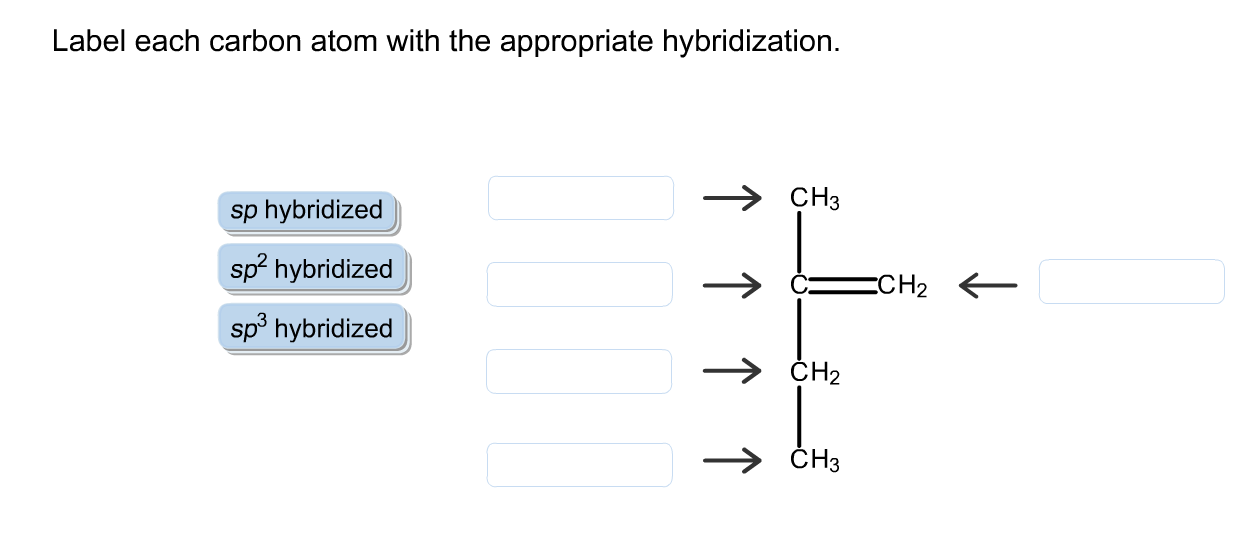
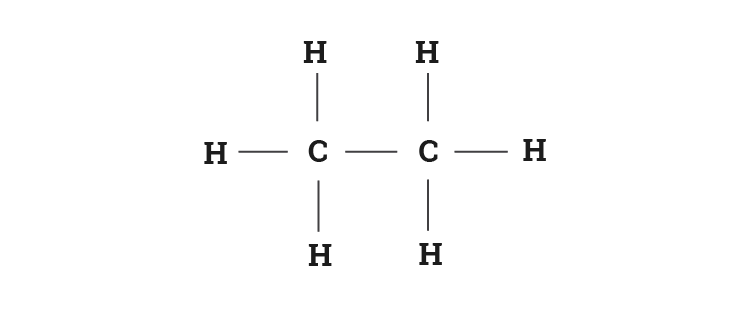
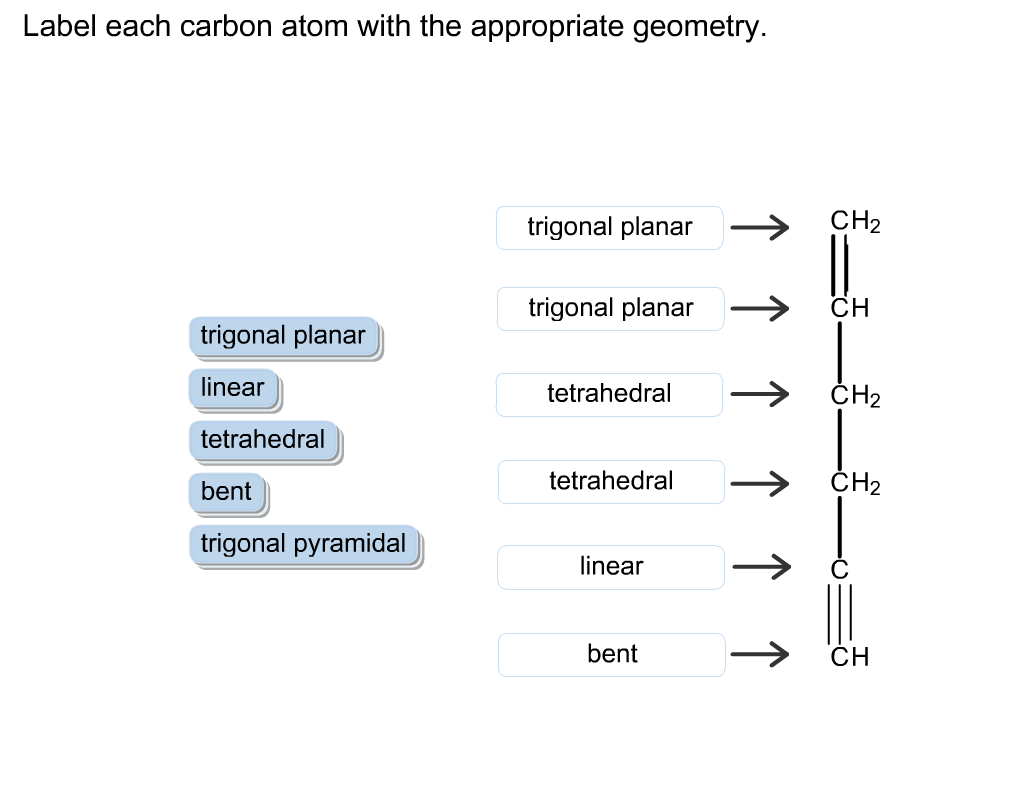
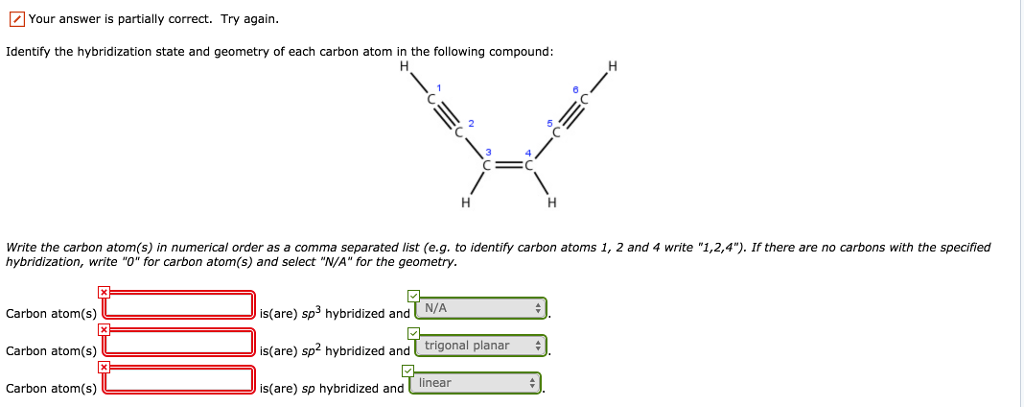
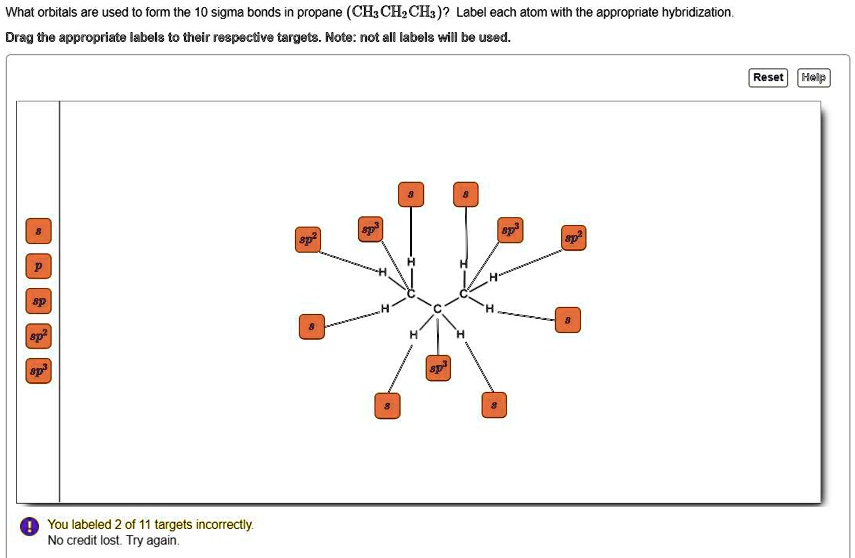
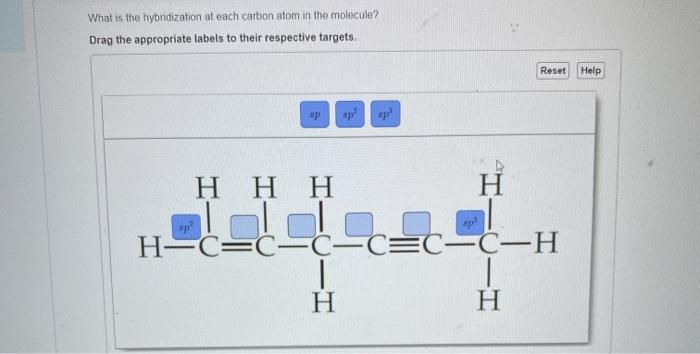
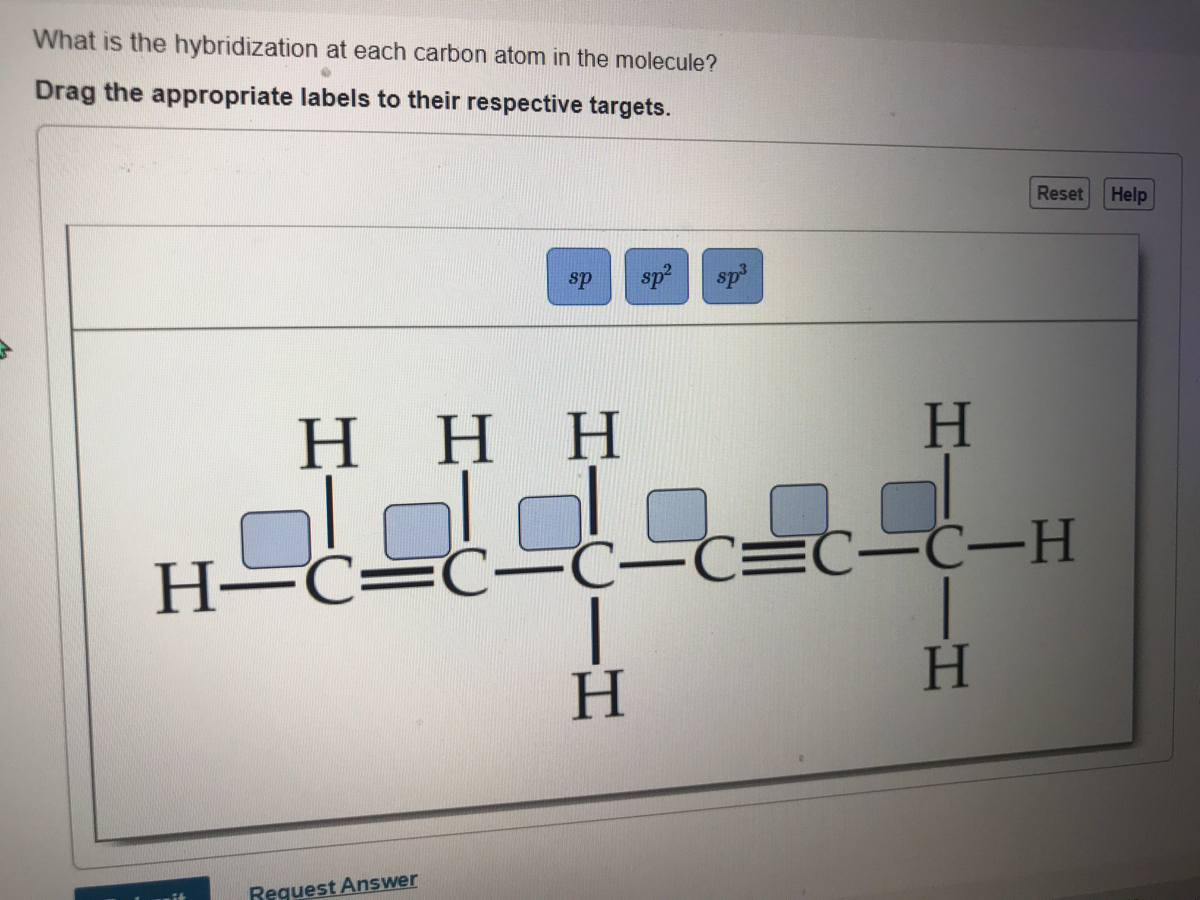
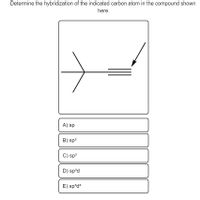
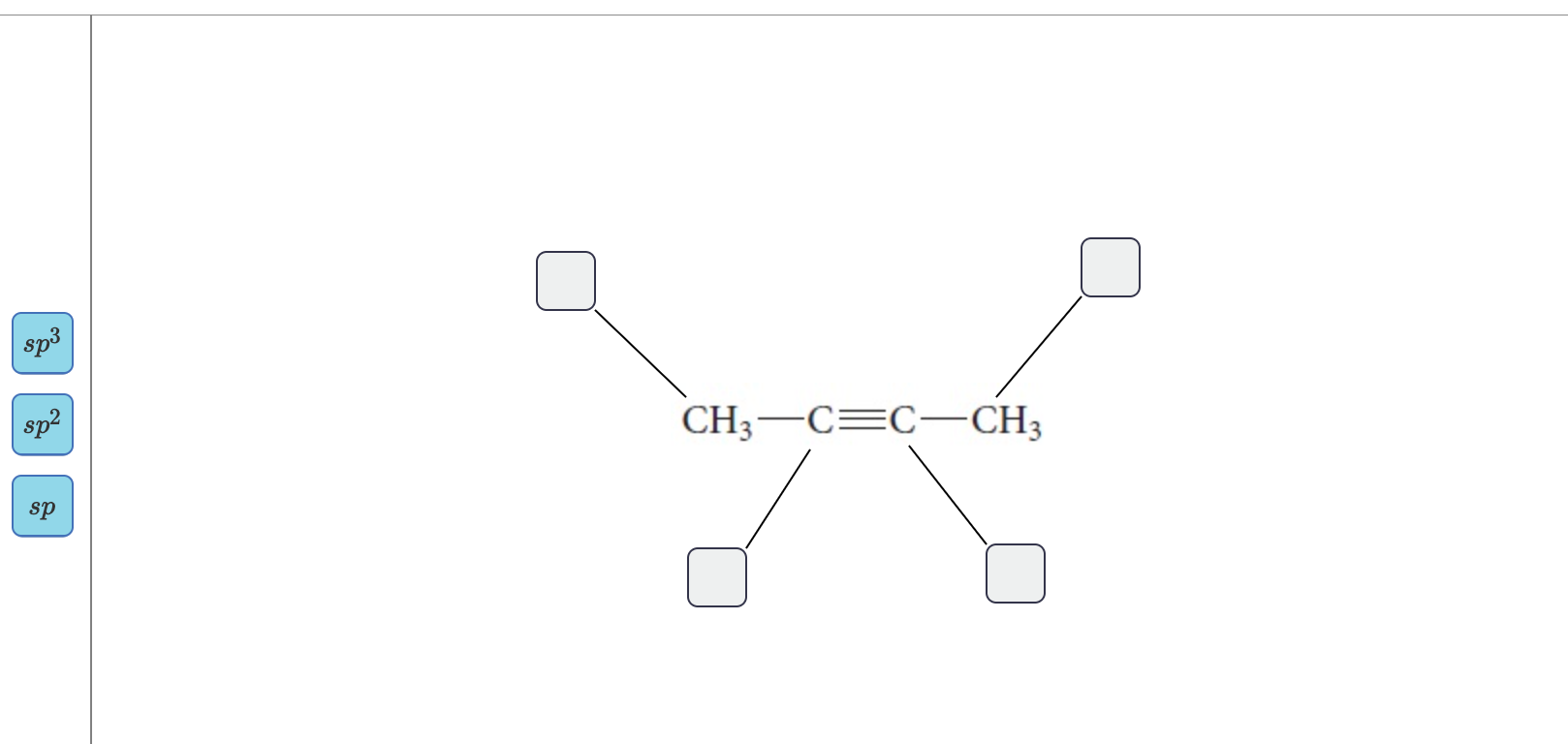
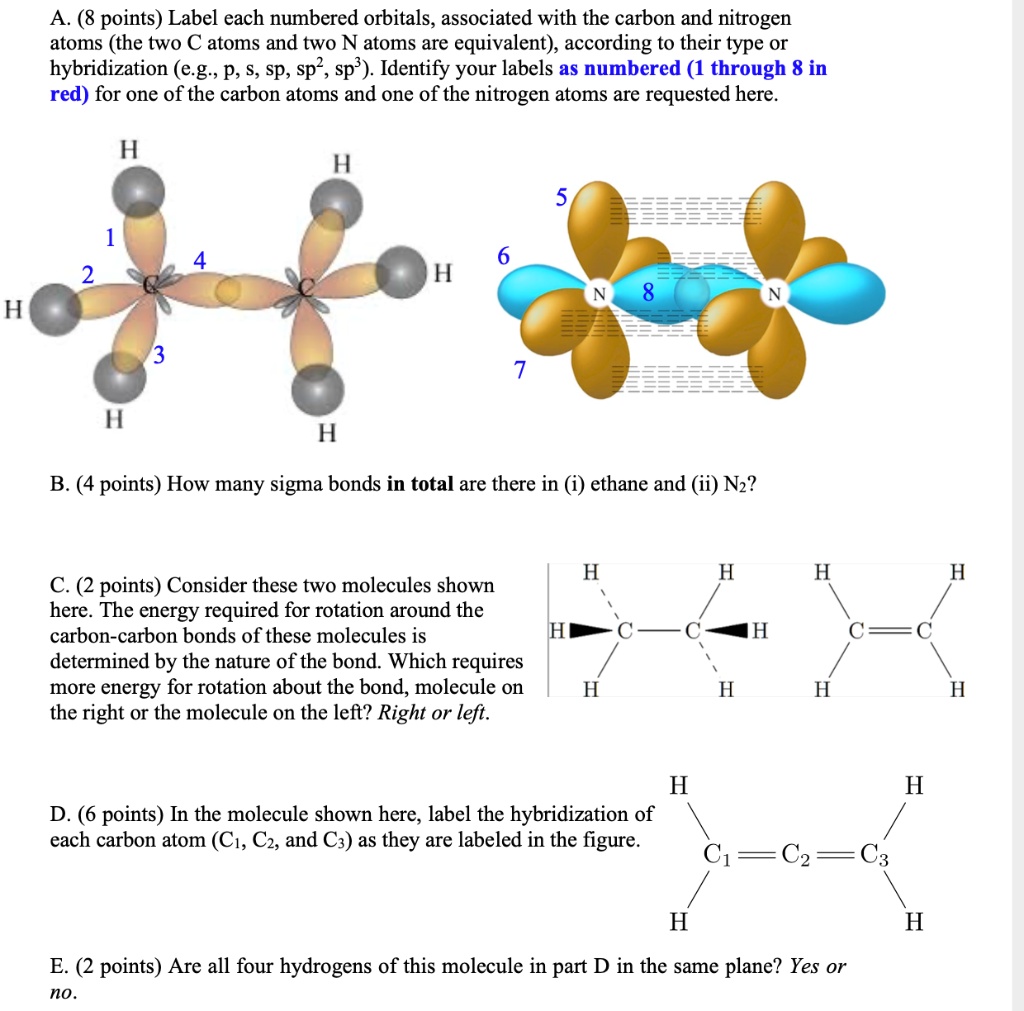
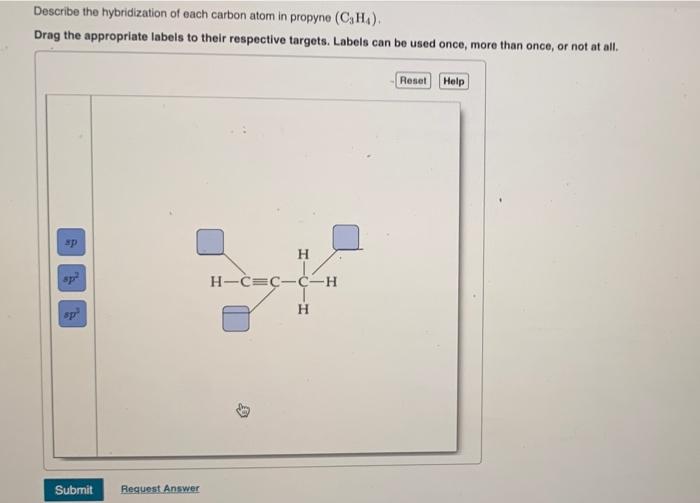
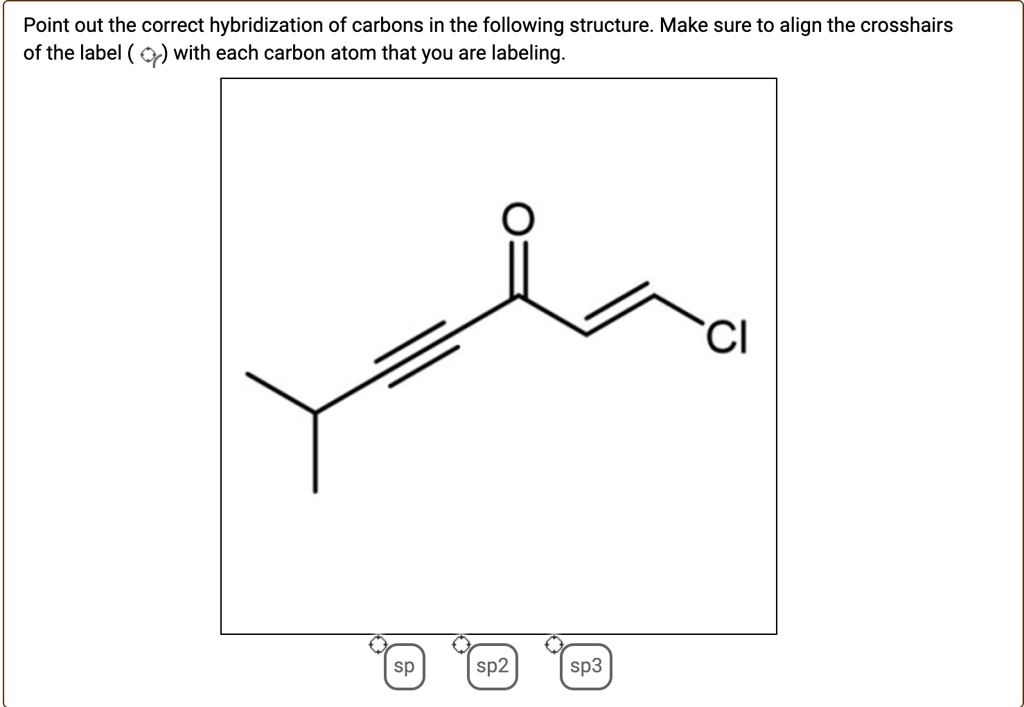
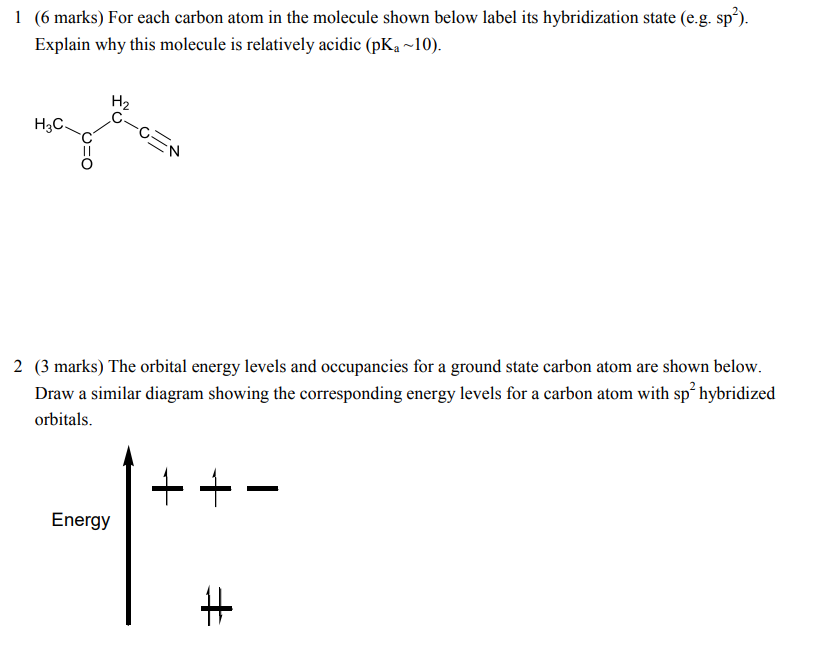
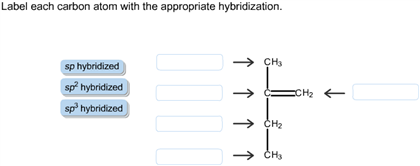
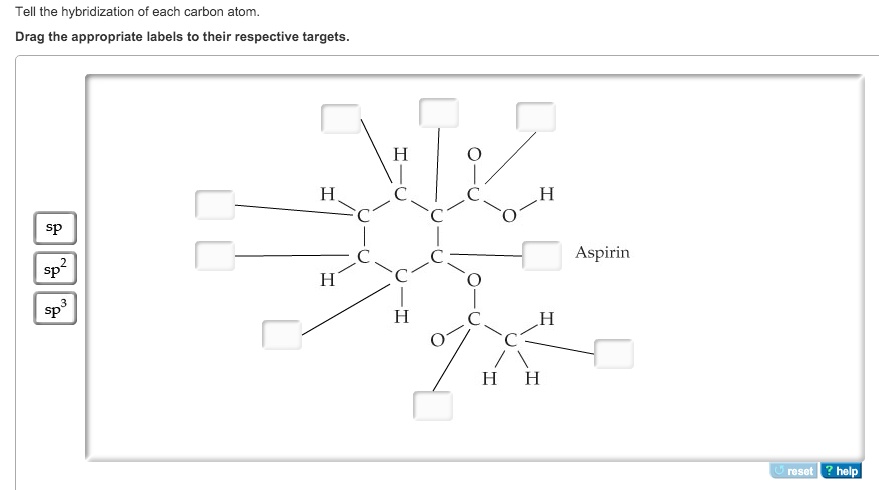
44 label each carbon atom with the appropriate hybridization
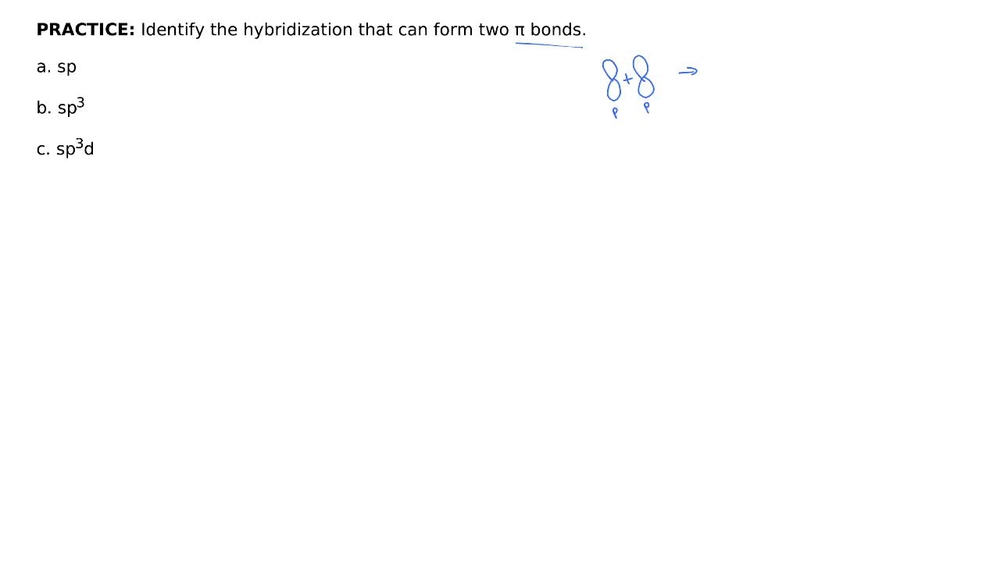
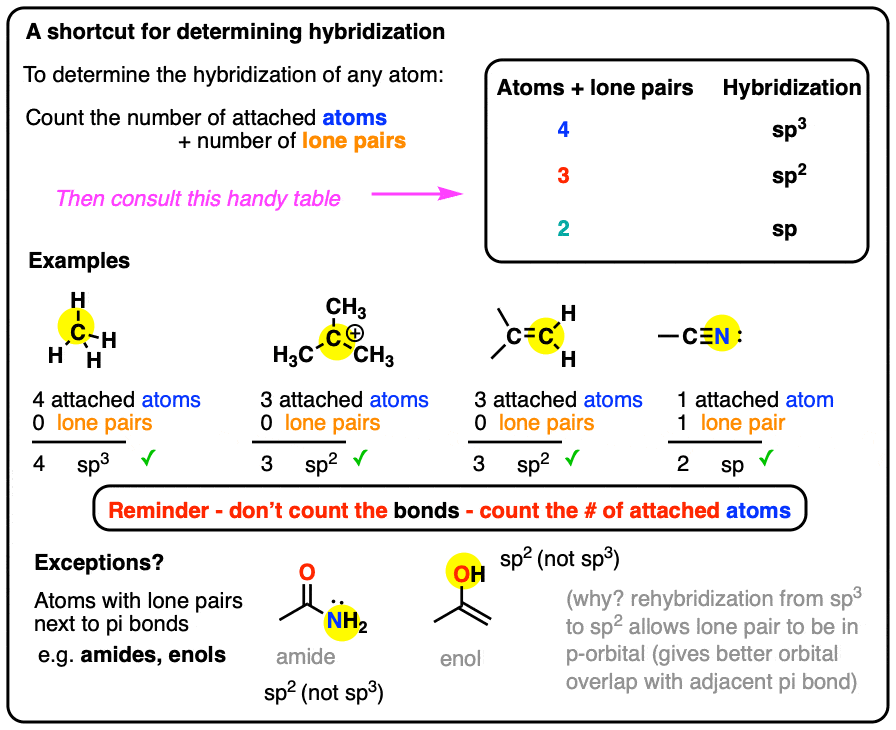
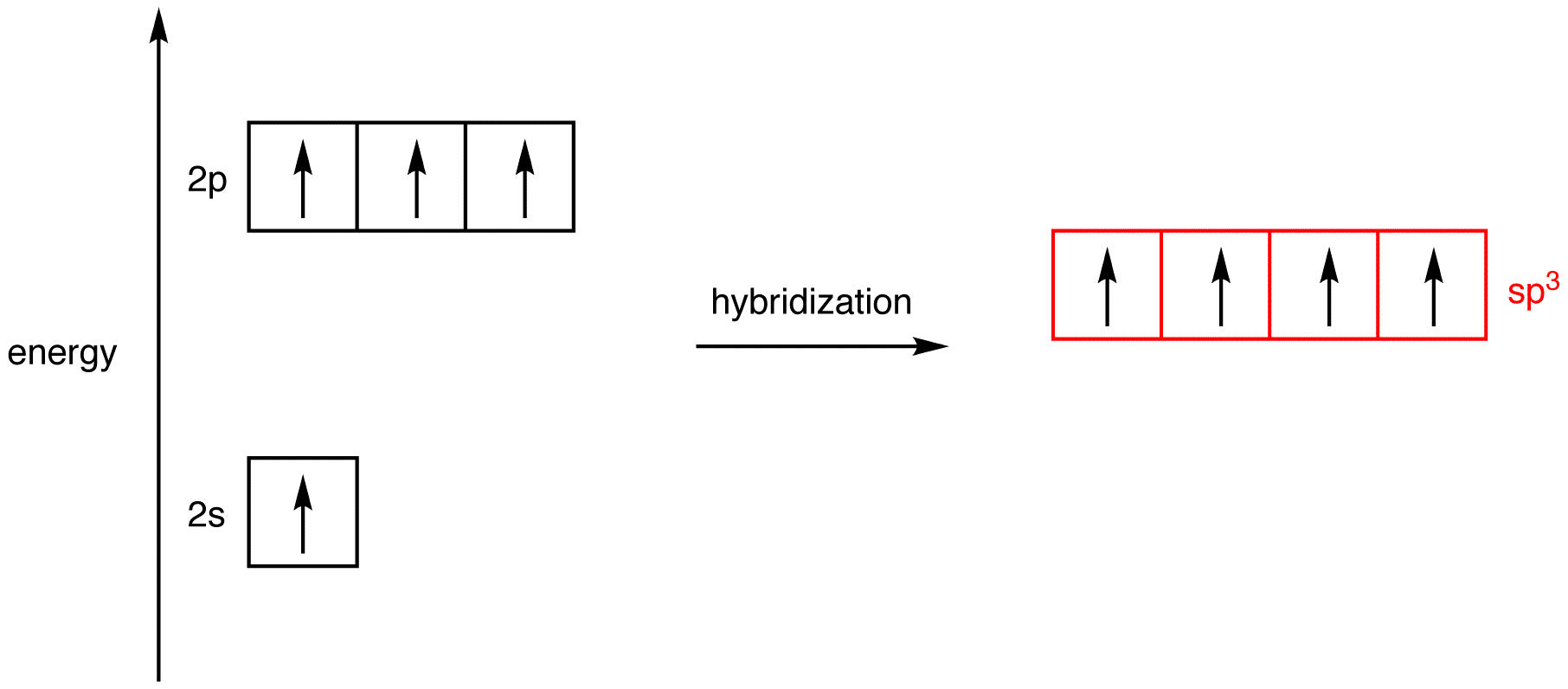
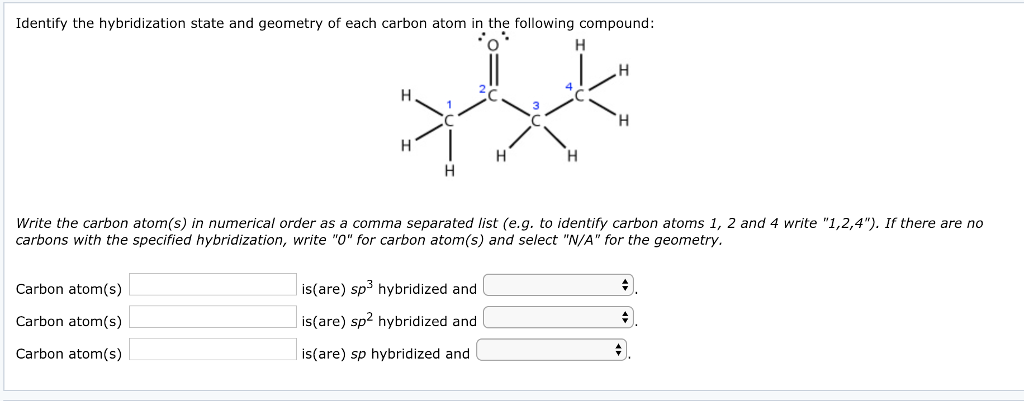
Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized Orbitals,... Try This: Give the hybridization states of each of the carbon atoms in the given molecule. H 2 C = CH – CN; HC ≡ C − C ≡ CH; H 2 C = C = C = CH 2; Types of Hybridization. Based on the types of orbitals involved in mixing, the hybridization can be classified as sp 3, sp 2, sp, sp 3 d, sp 3 d 2, sp 3 d 3. Let us now discuss the various ... sp³ hybridization | Hybrid orbitals | Chemical bonds (video) | ... Count the number of lone pairs + the number of atoms that are directly attached to the central atom. This is the steric number (SN) of the central atom. For example, the O atom in water (H₂O) has 2 lone pairs and 2 directly attached atoms. ∴ SN = 2 + 2 = 4, and hybridization is sp³.
OChem Spring 2017 Exam 1 Flashcards | Quizlet Draw the line structure of Butane. Carbon must have _____ bonds. 4. Draw the kekule structure of CH₃CHClCH₃. Draw the line structure of CH₃CHClCH₃. (2 configurations) Draw the kekule structure for CH₃CH (CH₃)CH₂CH₃. Name structure (cover right side of screen) Methane.

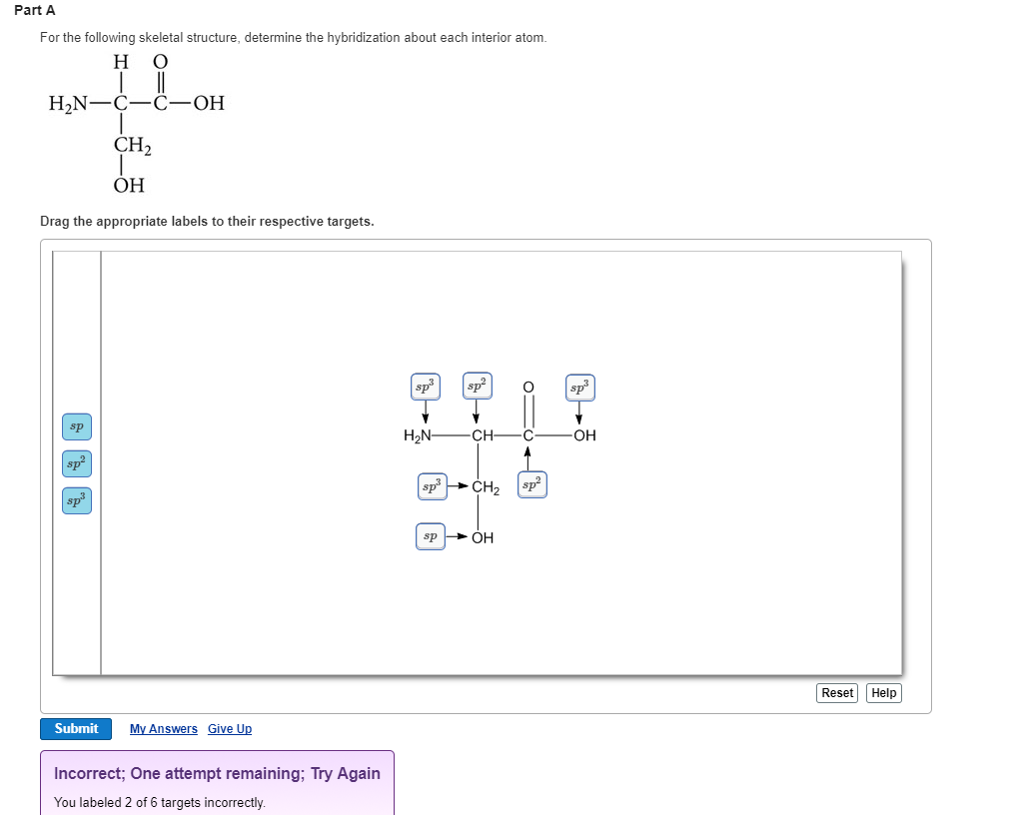
Label each carbon atom with the appropriate hybridization
label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... Hybridization of Carbon - Molecular Geometry and Bond Angles -... Carbon can have an sp hybridization when it is bound to two other atoms with the help of two ... Finding the hybridization of atoms in organic molecules (worked... There is no general connection between the type of bond and the hybridization for all molecules but since in organic chemistry it is only the hybridization of carbon which we need to know, we get the following connection-Single bonded carbon is sp3 hybridized. Double bonded carbon is sp2 hybridized. Triple bonded carbon is sp hybridized.
Label each carbon atom with the appropriate hybridization. Solved Label each carbon atom with the appropriate | Chegg.com Label each carbon atom with the appropriate hybridization. sp hybridized > CH3 > -CH2 { spr hybridized sp3 hybridized * * * * > CH2 > CH3 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Finding the hybridization of atoms in organic molecules (worked... There is no general connection between the type of bond and the hybridization for all molecules but since in organic chemistry it is only the hybridization of carbon which we need to know, we get the following connection-Single bonded carbon is sp3 hybridized. Double bonded carbon is sp2 hybridized. Triple bonded carbon is sp hybridized. Hybridization of Carbon - Molecular Geometry and Bond Angles -... Carbon can have an sp hybridization when it is bound to two other atoms with the help of two ... label each carbon atom with the appropriate hybridization 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...











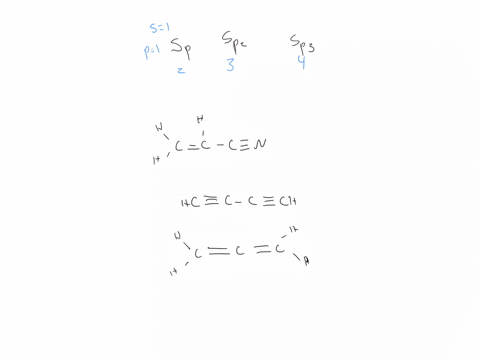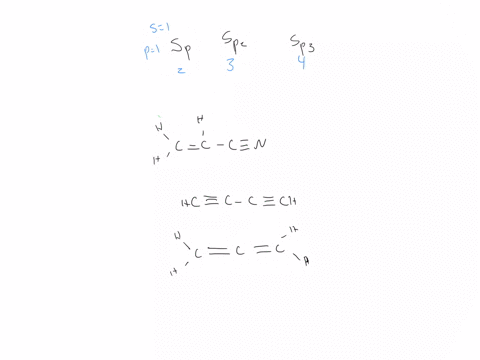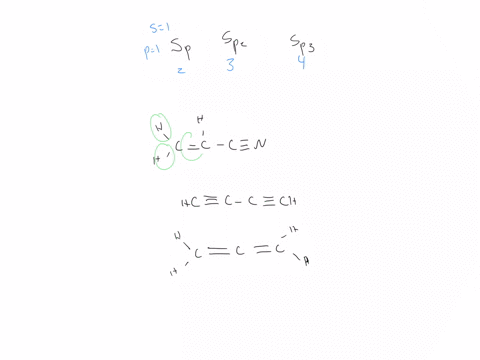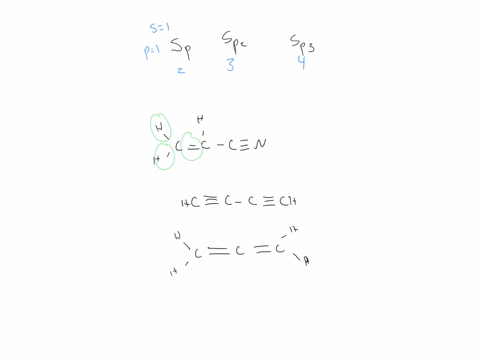




Komentar
Posting Komentar