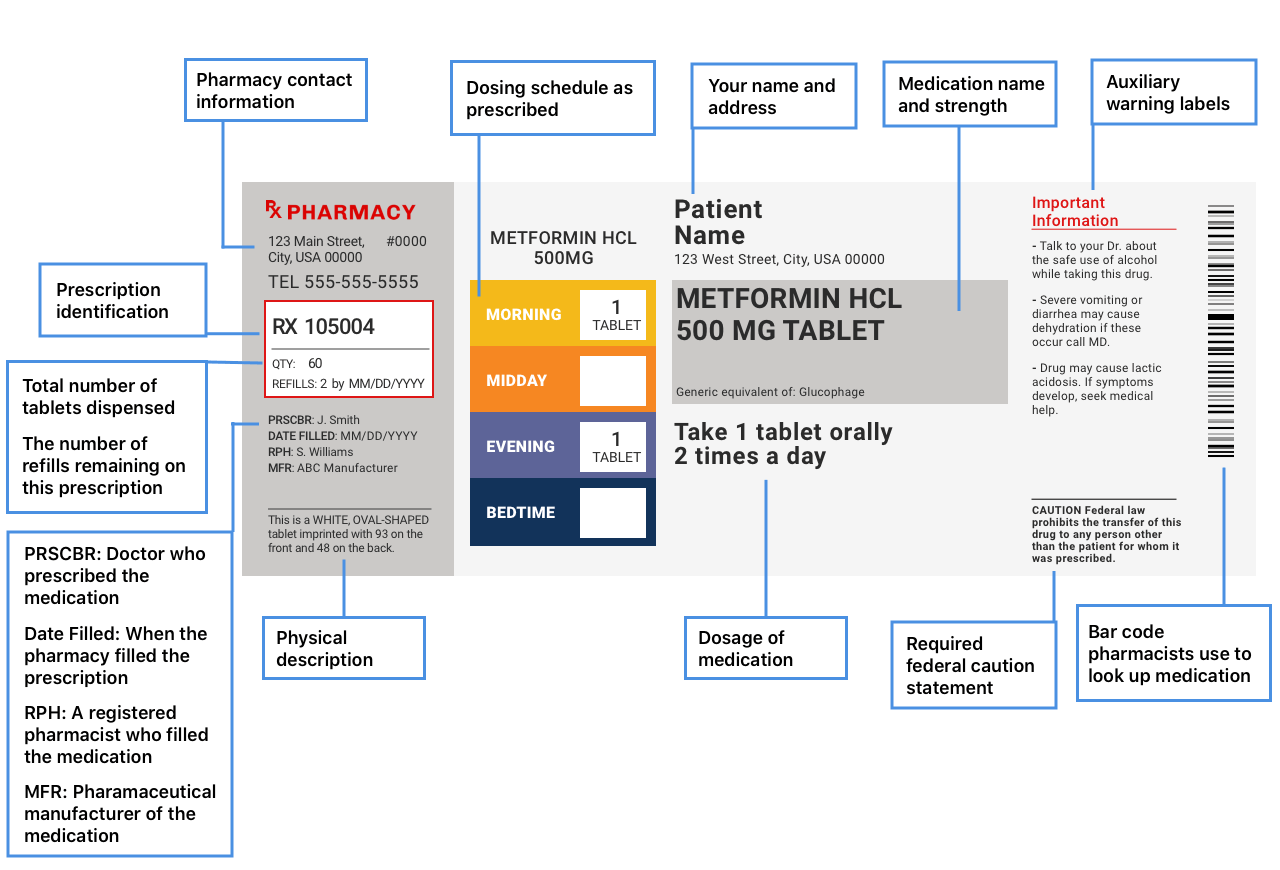
45 how to cite fda drug label
en.wikipedia.org › wiki › Combined_oralCombined oral contraceptive pill - Wikipedia The U.S. Food and Drug Administration (FDA) initiated studies evaluating the health of more than 800,000 women taking COCPs and found that the risk of VTE was 93% higher for women who had been taking drospirenone COCPs for 3 months or less and 290% higher for women taking drospirenone COCPs for 7–12 months, compared with women taking other ... › therapiesIvermectin | COVID-19 Treatment Guidelines Apr 29, 2022 · Ivermectin is a Food and Drug Administration (FDA)-approved antiparasitic drug used to treat several neglected tropical diseases, including onchocerciasis, helminthiases, and scabies. 1 For these indications, ivermectin has been widely used and is generally well-tolerated. 1,2 Ivermectin is not approved by the FDA for the treatment of any viral infection.
en.wikipedia.org › wiki › Food_and_Drug_AdministrationFood and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ...

How to cite fda drug label
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (i) If the label, labeling, or advertising of a food makes any direct or indirect representations with respect to the primary recognizable flavor(s), by word, vignette, e.g., depiction of a fruit, or other means, or if for any other reason the manufacturer or distributor of a food wishes to designate the type of flavor in the food other than ... en.wikipedia.org › wiki › Prescription_drugPrescription drug - Wikipedia The Food and Drug Administration (FDA) is charged with implementing the law. Misuse or abuse of prescription drugs can lead to adverse drug events, including those due to dangerous drug interactions. The package insert for a prescription drug contains information about the intended effect of the drug and how it works in the body. › scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (i) If the label, labeling, or advertising of a food makes any direct or indirect representations with respect to the primary recognizable flavor(s), by word, vignette, e.g., depiction of a fruit, or other means, or if for any other reason the manufacturer or distributor of a food wishes to designate the type of flavor in the food other than ...
How to cite fda drug label. en.wikipedia.org › wiki › Generic_drugGeneric drug - Wikipedia Generic drug names are constructed using standardized affixes that distinguish drugs between and within classes and suggest their action. [citation needed]Economics. When a pharmaceutical company first markets a drug, it is usually under a patent that, until it expires, the company can use to exclude competitors by suing them for patent infringement. › scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (i) If the label, labeling, or advertising of a food makes any direct or indirect representations with respect to the primary recognizable flavor(s), by word, vignette, e.g., depiction of a fruit, or other means, or if for any other reason the manufacturer or distributor of a food wishes to designate the type of flavor in the food other than ... en.wikipedia.org › wiki › Prescription_drugPrescription drug - Wikipedia The Food and Drug Administration (FDA) is charged with implementing the law. Misuse or abuse of prescription drugs can lead to adverse drug events, including those due to dangerous drug interactions. The package insert for a prescription drug contains information about the intended effect of the drug and how it works in the body. › scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (i) If the label, labeling, or advertising of a food makes any direct or indirect representations with respect to the primary recognizable flavor(s), by word, vignette, e.g., depiction of a fruit, or other means, or if for any other reason the manufacturer or distributor of a food wishes to designate the type of flavor in the food other than ...
.jpg)






![PDF] FDA pregnancy risk categories and the CPS: do they help ...](https://d3i71xaburhd42.cloudfront.net/84279827596fbdc7657c73efcf834cffc5c9232c/2-Table1-1.png)













![PDF] Toward Creating a Gold Standard of Drug Indications from ...](https://d3i71xaburhd42.cloudfront.net/c231e1899261c9d123b8e45ed53703ebbe47f74b/2-Figure1-1.png)








Komentar
Posting Komentar